EzriCare and Delsam Eye Drops Lawsuits
EzriCare eye drops have been linked to an outbreak of Pseudomonas aeruginosa, an antibiotic-resistant bacterial infection that has led to permanent vision loss, surgical eyeball removal, and death. If you or your loved one were harmed after using EzriCare or Delsam Pharma eye care products, Cutter Law P.C. can help you pursue significant damages through an EzriCare eye drops lawsuit.

EzriCare Artificial Tears, Delsam Pharma Artificial Tears, and Delsam Pharma Artificial Eye Ointment are over-the-counter brands of eye lubricants used for dry, irritated eyes. Global Pharma Healthcare has voluntarily recalled EzriCare and Delsam Pharma-branded products in response to a bacterial infection outbreak that first came to the attention of the United States Food & Drug Administration and the Centers for Disease Control & Prevention in May of 2022.
While working in cooperation with state and local governments, the CDC has identified a growing number of patients throughout the country who have become infected with an antibiotic-resistant strain of the bacteria Pseudomonas aeruginosa. This particular strain of P. aeruginosa has not previously been found in the United States.
Although the infection originates in the eyes, it can travel throughout the body and affect numerous organs. Health officials have detected the bacteria through blood, urine, and other bodily fluids cultured from the throat, ears, and rectum, in addition to the eyes. Patients who died did so within 30 days of using the drops.
If you have experienced an adverse reaction after using EzriCare or Delsa Pharma artificial eye drops and ointments, or if your loved one has passed away after using these products, you may be eligible to recover substantial compensation. The product liability lawyers at Cutter Law P.C. can help.
Quick Links
- EzriCare Lawsuit Updates and Timeline
- Which Eye Drops Have Been Recalled?
- Who Is Global Pharma Healthcare?
- Complications and Injuries
- What to Do If You Have Used EzriCare or Delsam Pharma Products
- Who Is Liable for Injuries Caused by EzriCare Eye Drops?
- Who Can File a Lawsuit?
- Types of Damages You Can Recover in an EzriCare Lawsuit
- You Can Trust Cutter Law to Get the Results You Deserve
- Contact Cutter Law to Start Your Claim
- Frequently Asked Questions
EzriCare Lawsuit Updates and Timeline
May 15, 2023: 81 patients in 18 states with P. Aeruginosa infections have been identified, an increase of 13 patients and two states since March 14, 2023. The additional states are Delaware and Ohio. Four patients have died.
May 8, 2023: The FDA issued Import Alert 66-40 for products imported by Global Pharma Healthcare with a Detention Without Physical Examination (DWPE) status because the company has not complied with Good Manufacturing Practices (GMPs).
This alert instructs and authorizes field agents at the border to automatically refuse admission of the products to prevent their distribution within the United States.
March 14, 2023: The CDC has identified 68 patients in 16 states with the P. aeruginosa infection linked to EzriCare Artificial Eye Drops. This includes the addition of the following states since the previous update:
- Illinois
- North Carolina
- Pennsylvania
- South Dakota
March 2, 2023: The FDA concluded its unannounced inspection of the Global Pharma factory in India, where EzriCare and Delsam Pharma products are produced, and cited Global Pharma Healthcare for multiple deficiencies in quality control, sterilization procedures, and contamination prevention.
February 24, 2023: Delsam Pharma voluntarily recalled the Delsam Pharma eye ointment, which is manufactured at the same facility as the EzriCare eye drops
February 20, 2023: The FDA initiated the unannounced inspection of the Global Pharma Healthcare factory in India.
February 4, 2023: The first lawsuit against the makers of EzriCare Artificial Tears was filed in federal court in Kentucky as a class action lawsuit by a patient injured by the EzriCare eye drops.
February 2, 2023: The FDA added Delsam Pharma Artificial Tears to an alert warning consumers not to purchase or use EzriCare eye drops.
February 2, 2023: Global Pharma Healthcare voluntarily recalled Artificial Tears Lubricant Eye Drops distributed by EzriCare and Delsam Pharma due to the potential for contamination.
January 31, 2023: The CDC began collaborating with the FDA and health departments at state and local levels to investigate an outbreak of P. aeruginosa. As of this date, 55 infected patients in 12 states had been identified, an increase of five patients since the last update, with the addition of the state of Wisconsin.
January 20, 2023: The CDC issued the first public warning about EzriCare eye drops.
January 19, 2023: The CDC completed its initial investigation of the P. Aeruginosa outbreak after identifying 50 patients in the following 11 states with the P. Aeruginosa infection:
- California
- Colorado
- Connecticut
- Florida
- Nevada
- New Jersey
- New Mexico
- New York
- Texas
- Utah
- Washington
May 17, 2022: The CDC initiated its investigation of EzriCare eye drops in partnership with state and local health departments and in cooperation with the FDA.
Which Eye Drops Have Been Recalled?
Global Pharma Healthcare has voluntarily recalled all the eye lubricants it manufactures. This includes products marketed and sold under the following names:
- EzriCare Artificial Tears
- Delsam Pharma Artificial Tears
- Delsam Pharma Artificial Ointment.
Who Is Global Pharma Healthcare?
Global Pharma Healthcare Private Limited is a drug manufacturer based in Tamilnadu, India. The products are imported into the United States from the manufacturing facility in India and distributed by domestic distributors.
Global Pharma Healthcare’s Distributors
The eye drops and ointment are distributed by Ezricare LLC, Delsam Pharma LLC, and Aru Pharma Inc. Ezricare and Delsam Pharma are private-label companies in the United States that contract directly with Global Pharma Healthcare to market and sell the products.
Aru Pharma distributes Ezricare-branded products. Ezricare LLC is based in New Jersey, while Delsam Pharma and Aru Pharma are located in New York.
Where Were the Products Sold?
Distributors typically do not sell directly to the public. Instead, they supply products to retailers at wholesale prices, who then offer them to the public at retail prices. Walmart and Amazon are the major retailers that sold EzriCare and Delsam Pharma products.
Global Pharma Healthcare Manufacturing Deficiencies
Since Global Pharma Healthcare manufactures medical products for distribution in the United States, it must abide by FDA regulations. The FDA imposes manufacturing standards to protect the safety and effectiveness of all drugs, including eye drops, and facilities that manufacture products are subject to inspections.
The FDA initiated an unannounced ten-day inspection of Global Pharma Healthcare’s manufacturing facilities on February 20, 2023, in response to the outbreaks. The results of this inspection were alarming.
The FDA found 11 major manufacturing deficiencies, including the following:
- Lack of quality control in sterilization processes, compliance procedures, and components of packaging materials, including caps and seals
- Inaccurate methods for testing product sterility
- Deficient cleaning and disinfection systems
- Failure to test and verify product components received from suppliers
- Lack of written policies and procedures for cleaning and maintaining equipment used to manufacture products
- Failure to monitor environmental contaminants
- Insufficient authority of the quality control unit within the company to approve or reject products
Essentially, the FDA found that Global Pharma Healthcare had failed to take the required measures to prevent contamination of its products during manufacture. These findings led the FDA to issue an import alert that requires all products imported by Global Pharma Healthcare to be refused admission without an inspection.
Although all of the products linked to the recall were marketed and sold by EzriCare, the Delsam Pharma products were also recalled because they were manufactured in the same factory and likely exposed to similar contamination risks.
Complications and Injuries
The contamination of eye drops manufactured by Global Pharma Healthcare with carbapenem-resistant P. aeruginosa has exposed American consumers to a bacterial strain never seen before in the United States.
This infection is extremely resistant to treatment, and the effects can be devastating.
Early Signs and Symptoms
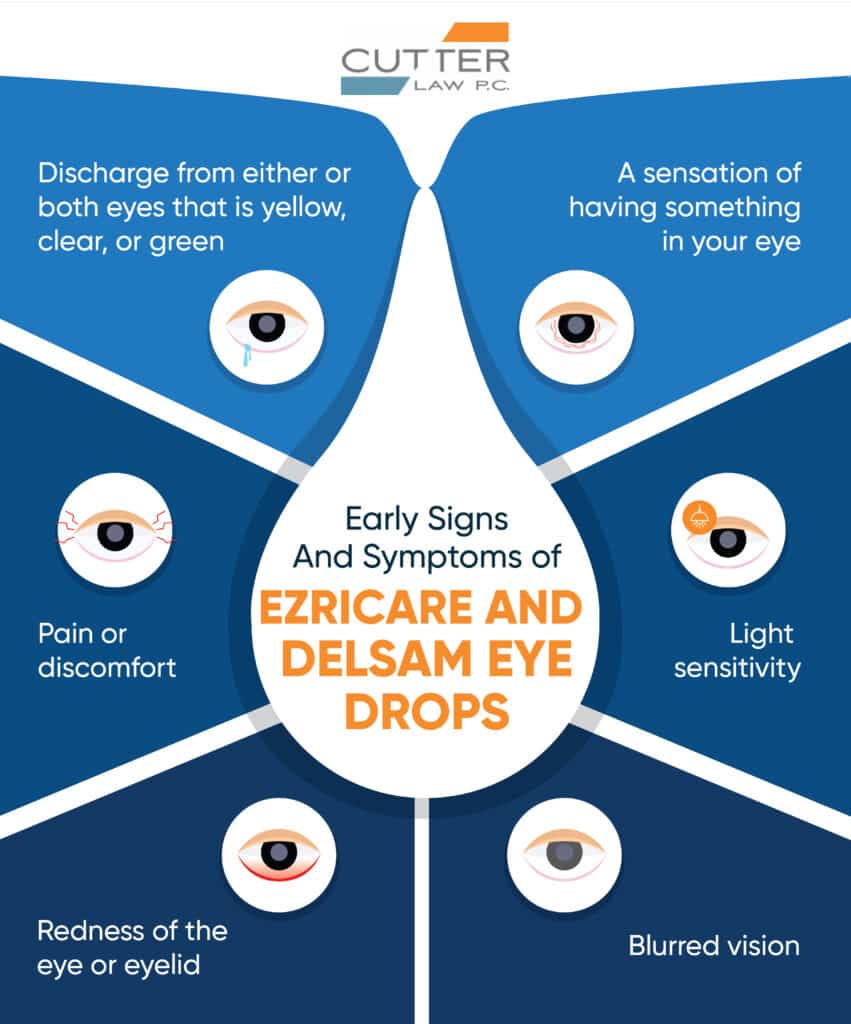
If you experience any of the following symptoms after using Ezricare or Delsam Pharma eye lubricant products, seek medical attention immediately:
- Discharge from either or both eyes that is yellow, clear, or green
- Pain or discomfort
- Redness of the eye or eyelid
- A sensation of having something in your eye
- Light sensitivity
- Blurred vision
As difficult as this infection is to eradicate, catching it as early as possible is crucial to give yourself the best chance of avoiding blindness or worse.
Long-Term Effects
During an interview with the Washington Post, Dr. Robert T. Schooley of the University of San Diego explained that the concentration of P. aeruginosa can be high enough to overwhelm the immune system, even in patients with strong ones. It also tends to attack vulnerable areas of the body where the immune response tends to be lower.
While the infection can cause severe, permanent effects in the eyes, it can also travel throughout the body. Effects observed in patients with the strain of P. aeruginosa found in the contaminated eye drops include the following:
- Vision loss
- Surgical removal of the eyeball
- Sepsis
- Respiratory tract infections
- Urinary tract infections
- Death
What to Do If You Have Used EzriCare or Delsam Pharma Products
If you are using EzriCare or Delsam Pharma products, the CDC recommends that you stop using them immediately and discard any product that you have left over. This warning applies to the following products:
- EzriCare Artificial Tears
- Delsam Pharma Artificial Tears
- Delsam Pharma Artificial Ointment
If you have any symptoms of an eye infection, you should seek medical attention immediately. If your health care provider instructed you to use eye drops or an ointment, you should seek advice from your physician about an alternative eye medication.
No testing recommendations currently exist for patients who have used the drops but have not developed symptoms.
If you are diagnosed with an infection related to EzriCare or Delsam Pharma eye drops or ointment, you are encouraged to report it to the FDA using the FDA’s adverse event reporting system or by contacting an FDA consumer complaint coordinator.
Do not discard the bottle if you have developed symptoms or suffered harm after using eye drops. Instead, secure it in a sealable bag and contact one of our product liability lawyers at Cutter Law as soon as possible. The bottle may serve as important evidence during your lawsuit.
Who Is Liable for Injuries Caused by EzriCare Eye Drops?
A lawsuit for a harmful product falls under product liability law, which makes manufacturers, distributors, retailers, importers, and anyone else in the supply chain strictly liable for harm that stems from using the product as directed. In this case, the following companies may be liable:
- Global Pharma Health Private Limited
- Aru Pharma Inc.
- EzriCare LLC
- Delsam Pharma LLC
- Amazon Inc.
- Walmart Inc.
Aru Pharma filed for Chapter 11 bankruptcy protection on February 27, 2023, partly in response to EzriCare lawsuits, according to the Westchester & Fairfield County Business Journals.
A successful Chapter 11 bankruptcy would shield Aru Pharma from lawsuits, but a court may require the company to set aside funds to compensate victims, depending on its available assets.
Who Can File a Lawsuit?
You may be eligible to file a lawsuit if you have used EzriCare or Delsam Pharma eye lubricant products and developed a P. aeruginosa infection. Lawsuits have already been filed for patients who have experienced severe effects from the infection, including permanent vision loss and surgical removal of the eyeball.
Our experienced product liability lawyers at Cutter Law can identify the responsible parties, gather the evidence you need, and help you pursue significant compensation.
Is There an EzriCare Class-Action Lawsuit?
While class-action lawsuits have been filed in some states, there is no nationwide class-action. As the number of cases continues to increase, the cases could ultimately go to multidistrict litigation.
Multidistrict litigation, or MDL, is a process used to resolve similar claims against a common defendant group. They are transferred to a single federal court. Unlike a class-action lawsuit, the cases remain separate, but discovery is consolidated. A few test cases are selected, and these go to trial.
These trials are known as bellwether trials. The results of the bellwether trials guide settlement negotiations in the remaining cases and may result in a global settlement.
Our product liability lawyers have experience handling class-action lawsuits, MDL cases, and all forms of complex litigation against large drug companies. We can help you determine the best venue and format to file your claim, and we know what it takes to get results.
Types of Damages You Can Recover in an EzriCare Lawsuit
The recoverable damages in a pharmaceutical liability case can be substantial, depending on the severity of your injuries and how they impact your finances and quality of life. Compensation for defective eye drops may include the following types of damages:
- Economic Damages
- Non-Economic Damages
- Punitive Damages
Economic damages are compensation for monetary losses, including medical expenses and lost wages. These damages include projected future lost wages and future medical expenses. If you are permanently disabled because of your injuries, this amount may be significant.
Non-economic damages are also known as pain and suffering. These damages compensate you for such intangible losses as the following:
- Physical pain
- Emotional distress
- Loss of society
- Loss of enjoyment of life
- Inconvenience
- Humiliation
- Disfigurement
Punitive damages are known as non-compensatory damages. Unlike economic and non-economic damages, they are not intended to compensate a victim but to punish a defendant whose conduct was outrageous, intentional, or grossly negligent.
Punitive damages may be awarded in product liability cases if the company knowingly provided a harmful product or committed fraud to conceal its knowledge of harm.
Although proving negligence is unnecessary n product liability cases, you can sue for negligence. Proving gross negligence may help you recover punitive damages. The conditions the FDA discovered at the factory may rise to the level of gross negligence, but a judge or jury must determine this.
Punitive damage standards vary by state, and such awards are not available in all states.
You Can Trust Cutter Law to Get the Results You Deserve
If you have experienced harm because of contaminated eye drops, you need an attorney with experience in complex litigation against large corporations. Our award-winning attorneys have over 130 years of combined experience and have recovered hundreds of millions of dollars for injured clients and their families.
Our consistent record of results has earned us the respect and admiration of our peers, who have recognized us with such prestigious honors as the following:
- Super Lawyers
- Best Lawyers in America
- Martindale-Hubbell AV Preeminent rating for attaining the highest level of professional excellence
- The American Association of Justice Leaders Forum

Our Consistent Record of Successful Results
Our product liability lawyers have taken on a broad array of cases with a long track record of remarkable case results.
$240 Million Settlement
Our founder Brooks Cutter played an important leadership role in litigation against Boston Scientific and Guidant over defective cardiac defibrillators. He represented 5,000 individuals and helped achieve a $240 million nationwide settlement.
$220 Million Settlement
After being selected for a leading role in an MDL case against Medtronic Sprint Fidelis for defective defibrillator leads, we helped engineer a $220 million settlement.
$1 Million Settlement
We obtained a $1 million settlement for a client who suffered burn injuries from an exploding e-cigarette.
Unprecedented Client Satisfaction
We consistently get results for our clients because we care about each client and provide attentive, personalized service. The quality of support and advocacy we provide is a frequent subject of the unsolicited feedback we regularly receive from our clients, including the following:
“Very friendly and professional. They really took the time to listen to my concerns and treated me with respect and compassion. They answered all my questions fully and made sure I had a firm grasp of what was going on every step of the way. I was very impressed by how they handled the case. I would recommend them to anyone!”
–Desiree Pierce
“They care about you and help you through the legal process with respect and empathy.”
–Anna Lisa Lopez
“Margot [Cutter] and her team went above and beyond to assist me. Their communication and professionalism far exceeded my expectations. I highly recommend working with them for a painless and stress-free experience.”
–Alison Grady
Contact Cutter Law to Start Your Claim
If EzriCare or Delsa Pharma eye drops harmed you, you deserve compensation. We are accepting cases in all 50 states, and you only pay if we win. Don’t leave money on the table. Contact Cutter Law today to schedule a free consultation.
Frequently Asked Questions
Below are answers to questions we frequently receive about EzriCare eye drops and filing a lawsuit.
What Is Pseudomonas Aeruginosa?
Pseudomonas aeruginosa is a group of bacterial infections resistant to most antibiotics. There are multiple strains of this bacteria. The strain linked to eye drops has not previously been seen in the United States. It is associated with eye infections but can spread throughout the body.
How Did the Eye Drops Become Contaminated?
While the exact mechanism of contamination remains unknown, it most likely occurred at Global Pharma Healthcare’s manufacturing facility. The bacteria stain found in the infections has not previously been observed in the U.S., indicating a foreign exposure source. In addition, the FDA inspection of the facility found that the product and its packaging materials were not protected from contamination.
Were EzriCare Eye Drops FDA-Approved?
The FDA did not evaluate EzriCare eye drops before they were marketed and sold. Over-the-counter medications do not have to be approved by the FDA if the products comply with policies and regulations.
In March of 2023, the FDA determined that these products do not comply with Good Manufacturing Practices. As a result, they cannot currently be marketed or sold in the United States.
What Do I Have to Prove in an EzriCare Eye Drops Lawsuit?
The EzriCare eye drops case is a product liability case, generally filed on the grounds of strict liability. In strict liability cases, it is not necessary to prove negligence. You only need to prove that you used the product as directed and suffered harm stemming from at least one of the following:
- A harmful product design
- A manufacturing defect
- Failure to warn or properly instruct
In this case, the product suffered from a manufacturing defect due to non-compliance with good manufacturing practices. Although proving negligence is unnecessary in a strict liability case, you can also allege negligence.
Can I Sue If My Family Member Died from the EzriCare Eye Drops Infection?
In the tragic event that your family member has passed away as a result of using EzriCare Eye Drops, your family may have the right to file a wrongful death lawsuit to recover compensation for the following:
- Your loved one’s medical expenses
- Funeral and burial expenses
- The lost wages of the deceased
- Your loved one’s pain and suffering
- The grief and suffering of your family
- Loss of companionship
- Loss of comfort
- Loss of guidance
- Loss of support
- Punitive damages
All types of wrongful death damages may not be available in all states. For example, some states do not allow damages for certain non-economic losses in wrongful death cases.
How Much Time Do I Have to File an EzriCare Eye Drops Lawsuit?
Every state imposes deadlines on filing lawsuits, known as a statute of limitations. In many states, this is one to three years after you become injured. Some states have separate deadlines for product liability cases based on the date you purchased the product. The time limit may also be different in wrongful death cases.
Statutes of limitations can be complex. It is best to speak with a knowledgeable lawyer as soon as possible after suffering an injury to determine how the statute of limitations applies to you.
Our experienced personal injury lawyers can help. It is crucial to contact us as soon as possible to ensure you meet this important deadline. Otherwise, you could lose your right to recover the compensation you need and deserve.
Testimonials
Schedule A Free Case Review
"*" indicates required fields
Our Office Locations
Sacramento Office
401 Watt Avenue Suite 100
Sacramento, CA 95864
Phone: 916-290-9400
Oakland Office
Cutter Law P.C.
1999 Harrison Street Suite 1400
Oakland, CA 94612








