California Medical Malpractice Lawyer
If you have been injured by a medical professional, you may be entitled to compensation. Medical malpractice cases can be difficult for many reasons, so you will need an experienced medical injury lawyer who can help you navigate the complex process.

Read more to find out about the process, the kinds of things you should expect, and why Cutter Law, the best medical malpractice law firm in California, is the right law firm for you.
Quick Links
Why Choose Cutter Law As Your California Medical Malpractice Lawyer?
If you need a Northern California medical malpractice lawyer, then Cutter Law has experienced local attorneys for you. From Sacramento to Oakland, our professionals have experience with major health systems, such as Kaiser, and are ready to help.
We have achieved success for medical malpractice clients, including $2.4 million for the children of the victim of intentional medical misconduct. Our California personal injury lawyers are ready to answer your questions during a free consultation.
$240 Million
Trial attorney Brooks Cutter took a lead role in a nationwide case against Boston Scientific and Guidant following a recall of the manufacturer’s pacemakers and cardiac defibrillators.
$7.6 Million
Medical malpractice jury verdict on behalf of a young woman who suffered a spinal cord injury after doctors failed to diagnose a tumor on her spine.
$ 2.4 Million
Brooks Cutter and Margot Cutter won a $2.4 million dollar verdict on behalf of the children of a man who died after a staff doctor at a hospital lied and transferred a patient to another hospital that was not equipped to handle the patient.
$220 Million
Appointed by the federal court to the plaintiffs steering committee in the Medtronic Sprint Fidelis case on behalf of patients injured by defective defibrillator leads, ultimately ending in a successful settlement for our clients.
What qualifies as medical malpractice in California?
Modern medicine has come a long way, but it is not yet perfect. Getting an outcome different than the one you were hoping for does not mean that you have experienced medical malpractice. To qualify as medical malpractice, your situation must meet certain requirements:
- A physician-patient relationship existed. You must have actually been a patient of the physician before you can sue for medical malpractice. That means that both you consented to the physician treating you, and the physician agreed to treat you. In most cases, the relationship is easy to prove because you likely signed a “consent for treatment” before the physician treated you. In some situations, the physician-patient relationship can be murky. A common situation is when a physician merely consulted another physician about your case.
- The physician did not meet the standard of care. The standard of care is what a reasonable physician of the same specialty in the same local area would have provided. Meeting the standard of care does not mean that a physician must have provided cutting-edge treatment or the same treatment that a world-renowned specialist may have provided. But if another doctor in your area would have done something differently, then you may have a case.
- You suffered a medical injury because of the physician. Unfortunately, even in the best circumstances, patients do not always get the best medical outcome. To establish medical malpractice, you have to be able to prove that, more likely than not, a physician’s action or inaction either caused a negative outcome for you or reduced your chances of getting a better outcome.
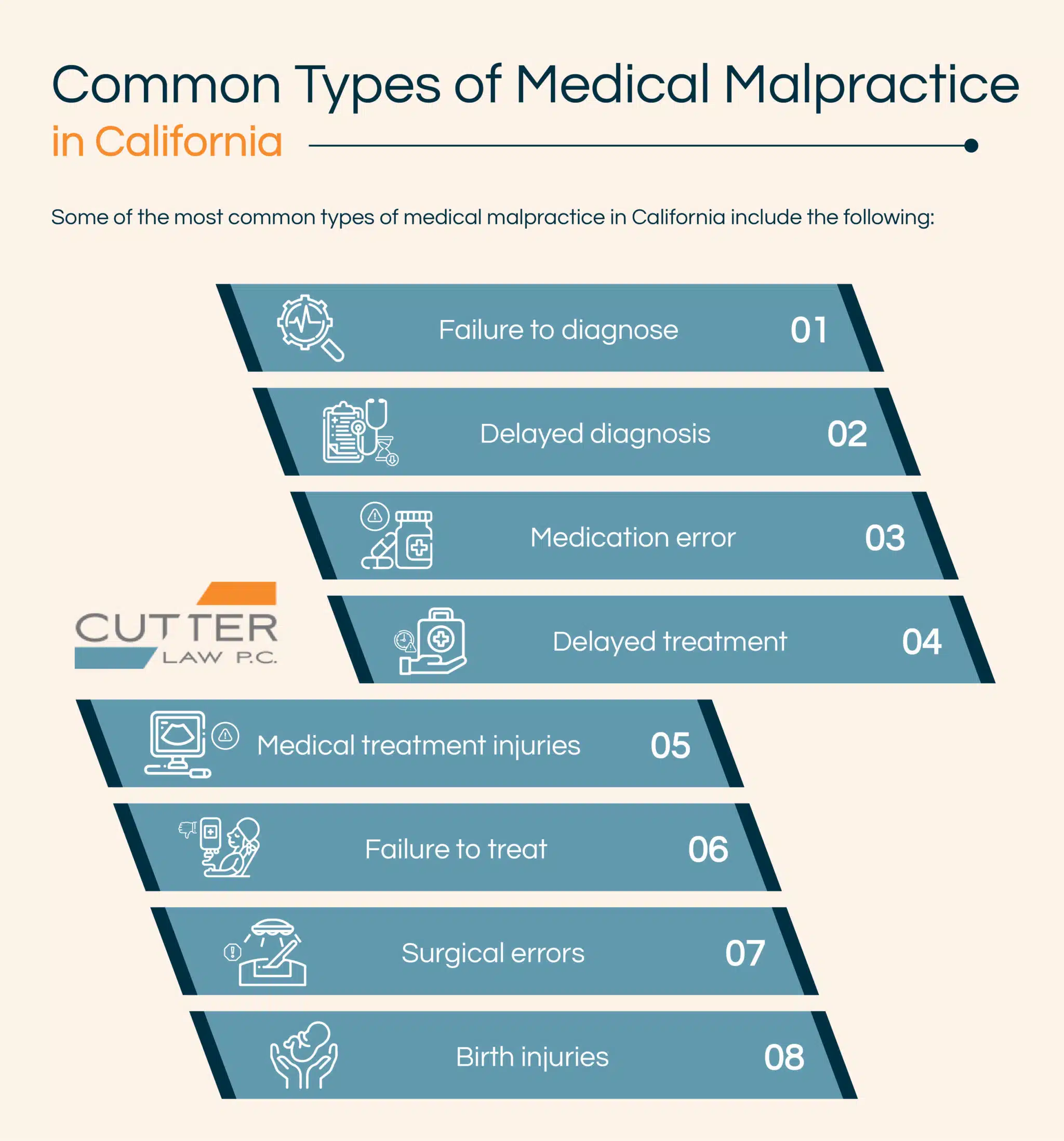
There are different types of medical malpractice. Some of the most common types of medical malpractice include the following:
The Process for a Medical Malpractice Case
The steps of a medical malpractice case are often dependent on the circumstances of your injury. Settlement discussions may occur at different stages, and not all medical malpractice cases will go to court. Some healthcare providers require you to sign an arbitration clause, which means your claim will go to private arbitration instead.
However, if your case does go to court, it will likely follow these steps:
Pre-Litigation Investigation and Evaluation
Before you begin the process of filing a medical malpractice case, a California medical malpractice attorney should first help you determine whether you meet the statute of limitations — the time limit you have to file a medical malpractice lawsuit. In most cases in California, you must file your suit before whichever of the following occurred first:
- One year after the patient discovered the injury
- Three years after the date the injury occurred
Before filing the suit, your attorney will also help you initially investigate your case. This may mean requesting medical records or other evidence. You will almost always need to find an expert witness to determine the appropriate standard of care and whether it was properly met by your treating provider. The expert witness is usually a physician or provider of the same specialty as your treating provider.
Notice and Filing Suit
California requires that a patient give the medical provider 90 days’ notice before the malpractice suit can be filed. The notice must include information about all of the following:
- The legal basis of your claims
- The type of loss you sustained
- The nature of the injuries you suffered
After the 90 days expires, you can file the suit. If you signed an arbitration agreement, the process to initiate the arbitration may be different.
Discovery and Pre-Trial Motions
Once your suit is filed, you will typically begin a discovery phase, when you request various types of evidence from the other party. An experienced medical injury attorney will know what types of documents to request as well as which witnesses to take depositions from. The defendant’s medical provider may request documents from you and take a deposition of you or your expert witnesses. Your attorneys may also file pre-trial motions at this time.
If your case must be arbitrated, you will likely still go through the discovery and pre-trial motion process, but it may be limited.
Trial or Arbitration
If your case goes to trial, you will almost certainly have to testify. Your expert witnesses, the treating medical providers, and other expert witnesses will likely testify as well. A judge will preside over the case, and either a judge or jury will decide whether the medical providers are liable for your injuries, as well as how much they must pay you as compensation.
If you signed an arbitration agreement, as most Kaiser patients are required to do, you will want a Kaiser malpractice attorney to represent you at the arbitration. An arbitration is similar to a court trial but generally less formal and with relaxed rules. Instead of a jury, an arbitrator selected by the parties determines whether the provider is liable and the award amount.
Appeals
If you or the providers disagree with the final ruling, either because the court found the providers not liable or you do not think your award was high enough, you can file an appeal. The appeals process can be very long and may include multiple appeals.
If you had to arbitrate your claim, then you have a very limited right to appeal.
Settlement
Depending on your case, your attorney may negotiate a settlement. Often patients do not want to go through the expense, time, and emotional toll of a trial and potential appeals. A settlement can be both quicker and more cost-effective.
Related: Guide to California Medical Malpractice Cap Changes

Liability in a Medical Malpractice Case
In medical malpractice cases, many parties can be held liable, including:
- Your treating physicians
- Physicians and providers who supervised or conducted any testing or screenings
- Physicians who were consulted about your care
- Emergency Department physicians who chose not to treat you
California is a comparative negligence state. That means that if you had any fault in your injuries, then your award is reduced by the percentage that you were at fault. For example, say a jury decides that your medical injury was 30% caused by your own negligence because you delayed seeking treatment and 70% by your physician’s medication error. If your injuries cost you $100,000 in damages, then your award would be reduced by 30% to $70,000.
California Medical Malpractice FAQs
What Should I Do If I Think A Doctor Committed Medical Malpractice?
Contact an attorney immediately to have your case evaluated. The time limits for filing malpractice claims in California are very short. Do not delay in seeking legal advice. Contact Cutter Law to schedule a free case review today.
How Do I Find The Best Medical Malpractice Lawyer in California?
To find the best attorney, evaluate the attorney’s track record and experience, and decide whether the attorney will devote the time and attention to your case that you deserve.
How Much is My Medical Malpractice Case Worth?
How much your case is worth depends on a number of factors. To receive an estimate, a California medical malpractice attorney needs to evaluate your case.
Contact our California medical malpractice lawyers at (916) 290-9400 if you have been injured due to the action or inaction of your medical provider. We help clients throughout California and have local offices in Sacramento, Oakland, and Santa Rosa.
The skilled legal team at Cutter Law will work on your behalf to get you the compensation you deserve.
Testimonials
Schedule A Free Case Review
"*" indicates required fields
Our Office Locations
Sacramento Office
401 Watt Avenue Suite 100
Sacramento, CA 95864
Phone: 916-290-9400
Oakland Office
Cutter Law P.C.
1999 Harrison Street Suite 1400
Oakland, CA 94612








